Why dietary management is critical in PKU
Phenylketonuria (PKU) is an inborn error of amino acid metabolism that affects around one in every 24,000 newborns globally1. Patients with PKU have an enzyme deficiency which reduces the body’s ability to metabolise phenylalanine (Phe), an essential amino acid naturally abundant in most dietary proteins. If PKU is not diagnosed and treated from an early stage, dietary Phe intake may cause severe intellectual disability, epilepsy and behavioural problems. Therefore, a diet low in Phe remains a key tool for PKU management while ensuring a high protein intake2.
Challenges of protein substitutes in PKU
A PKU diet is based on low-protein foods combined with protein substitutes. Dietary adherence is a complex challenge for individuals and families3. Protein substitutes are often based on artificial free amino acids which are associated with a bitter flavour and unpleasant aftertaste which might lead to poor compliance4. According to the European 2025 guidelines, free amino acids also have a lower biological efficiency than natural protein sources, which is one of the reasons why PKU patients may require up to 40% additional protein from low-Phe protein substitutes2. This makes it even more important to offer palatable low-Phe protein substitutes for the patients.
In contrast to free amino acids, casein glycomacropeptide (CGMP) – a polypeptide naturally low in Phe and derived from cheese whey – has a neutral taste and odour that improve palatability2. Over the years, substantial research has documented the benefits of CGMP in PKU nutrition compared to free amino acids5. In commercial protein substitutes, Lacprodan® CGMP-20 can partially replace free amino acids and provide additional health benefits, such as improved gastrointestinal comfort which has been shown in a clinical trial6.
Developing safe and effective proteins for PKU nutrition
Arla Foods Ingredients collaborates with leading scientific and nutritional experts to develop protein substitutes with a higher biological efficacy. Lacprodan® CGMP-20 has, consequently, become widely recognised as a suitable protein source in PKU management. Hence, the European 2025 guidelines state that “CGMP can be prescribed as a protein substitute in patients with PKU ≥4 years”2.
Daily Phe allowance should be considered at all times and is evaluated individually for all patients. Even the small amount of Phe in Lacprodan® CGMP-20 may challenge blood Phe control in those with the lowest levels of tolerance when used as the only protein substitute6. With ~50% less Phe than CGMP-20, Lacprodan® CGMP-30 may be a suitable solution in more sensitive patients, potentially supporting gastrointestinal comfort and better Phe control6.
CGMP is suitable for other inborn errors of amino acid metabolism
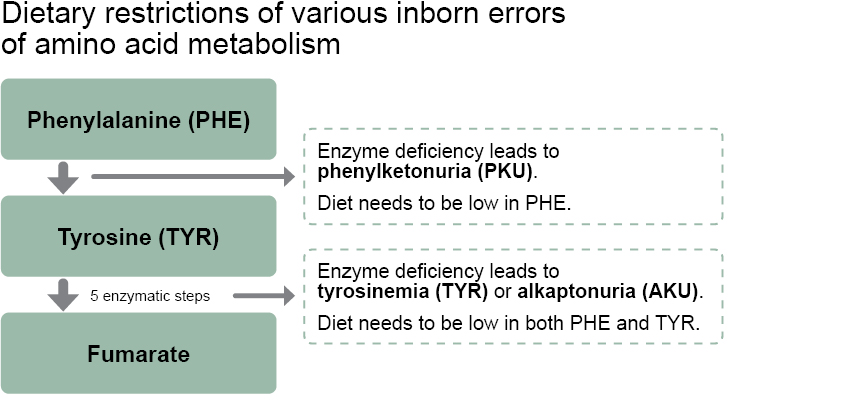
Due to the low content of Phe and tyrosine, Lacprodan® CGMP is also a suitable protein ingredient for managing tyrosinemia (TYR)7 and alkaptonuria (AKU)8 – two other inborn errors of amino acid metabolism (see figure).
References:
- Hillert, A., et al., The Genetic Landscape and Epidemiology of Phenylketonuria. Am J Hum Genet, 2020. 107(2): p. 234-250.
- van Wegberg, A.M.J., et al., European guidelines on diagnosis and treatment of phenylketonuria: First revision. Molecular Genetics and Metabolism, 2025. 145(2): p. 109125.
- Yagudina, R., et al., Factors Affecting Adherence to a Low Phenylalanine Diet in Patients with Phenylketonuria: A Systematic Review. Nutrients, 2024. 16(18): p. 3119.
- Tosi, M., et al., Glycomacropeptide-Based Protein Substitutes for Children with Phenylketonuria in Italy: A Nutritional Comparison. Nutrients, 2024. 16(7): p. 956.
- Daly, A., et al., Casein glycomacropeptide in phenylketonuria: does it bring clinical benefit? Curr Opin Clin Nutr Metab Care, 2024. 27(1): p. 31-39.
- Pinto, A., et al., The effects of casein glycomacropeptide on general health status in children with PKU: A randomized crossover trial. Molecular Genetics and Metabolism, 2024: p. 108607.
- Daly, A., et al., Casein Glycomacropeptide: An Alternative Protein Substitute in Tyrosinemia Type I. Nutrients, 2021. 13(9): p. 3224.
- Judd, S., et al., Evaluation of a casein glycomacropeptide‐based protein substitute, in the dietary management of NTBC‐induced tyrosinaemia in patients with alkaptonuria: A prospective open‐label study. Journal of Human Nutrition and Dietetics, 2024. 37(6): p. 1496-1504.







